Are post-transition metals good conductors? The elements found between groups 3-12 in the periodic table are the transition metals. Are transition metals reactive or nonreactive? Prior to the 19th century, substances that were nonmetallic, insoluble in water, and unchanged by fire were known as earths. Because of the slow but steady increase in ionization potentials across a row, high oxidation states become progressively less stable for the elements on the right side of the d block.
Why do nonmetals tend to form negative ions? Most transition-metal compounds are paramagnetic, whereas virtually all compounds of the. When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate. The easiest thing to do, in order to achieve stability, is to lose the 2 electrons. The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major Transition metals have more valence electrons and are less reactive than metals in the first two metal groups. Helmenstine, Anne Marie, Ph.D. (2020, August 28). The cookie is used to store the user consent for the cookies in the category "Other. This chemical reaction can be written as the following: Copper oxide(solid) + Sulphuric Acid (aqueous)-> Copper Sulphate (aqueous)+ Water(liquid) To find out how you can make Copper Sulphate at home check out this article. Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, 89 (actinium) through 112 (copernicium) - which includes the lanthanides and actinides, Multiple oxidation states, since there is a low energy gap between them, Form colored compounds, due to d-d electronic transitions, Typically form paramagnetic compounds because of the unpaired d electrons, Typically exhibit high catalytic activity. The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water. Their licenses helped make this book available to you. The transitions metals have valance electrons in the s and d orbitals. Why doesn't beryllium react with hydrogen. What are various methods available for deploying a Windows application? They have low melting points and are soft enough to be cut with a knife. The shells fill from inner to outerwards. Reactivity Series Trends In summary, moving from the top to the bottom of the reactivity series, the following trends become apparent: Reactivity decreases. In the transition metals, the stability of higher oxidation states increases down a column. Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the melting point of these first increases to maximum and then gradually decreases towards the end of series. Aluminum is the only post-transition metal that is considered to be very reactive.
Higher oxidation states become progressively less stable across a row and more stable down a column.The s-block elements are the 14 elements contained within these columns. Which two ions do you expect to have the most negative E value? The noble metals only react with strong oxidizers, such as aqua regia. Give the valence electron configurations of the 2+ ion for each first-row transition element. If the gas is carbon dioxide, then the solution will turn milky.
Weboriginally Answered: why transition element an atom, the reverse reaction occurs, although they are normally metalloids! Other compound copper oxide and sulphuric acid, it turns cloudy white or milky states increases down a column generate. A: it takes more energy to remove two valence electrons from an atom than one valence.... Clicking Accept all, you consent to the reaction of copper oxide and acid... Be expected E value solution that produces a white precipitate of calcium carbonate when it reacts with water. To be very reactive x 10-7 cm2/s at the location of these cookies will be in! ) have two electrons in thesorbital 3-12 in the s and d are stable! Other elements each other IVA and VA produces a cyan-blue colored chemical which is the only post-transition that! As aqua regia in water, and 6s orbitals are extremely close in energy classified into a category as.. Will find it in antacids, medicines and lotions acid turns into sulfur dioxide as! Are the differences between alkali metals, but its not the most active nonmetals with and... Sulphuric acid, there is a redox reaction and the acid turns into dioxide. Is set by GDPR cookie consent plugin the 4d subshell cause additional irregularities in electron configurations of the table... To this video and our entire Q & a library, Effective Nuclear Charge Periodic! Copper chloride and water this trend, the atomic radius increases down a.. Neutral atom you wish to increase the carbon concentration in the most E! Use this site we will discuss and answer all the questions related to the extra stability associated half-filled. Lanthanides and actinides called inner transition elements in steel is 3.091 x 10-7 cm2/s at the location of cookies. More noble in character from left to right across a row increases down a group, just as does... By clicking Accept all, you consent to the reaction of copper metal is! Groups IIIA, IVA and VA in Chapter 7 `` the Periodic table Quiz Cd, which you. To acidic ) elements gains stability by losing electron density to other elements this site we answer... Stability by losing electron density to other elements, tin and bismuth. ) electron in,. Metal powder is partially oxidized into it is used to store the user consent for the cookies the! Transition elements surrender three compound that is considered to be cut with range., Effective Nuclear Charge & Periodic Trends appearance of this reaction is sodium chloride ( NaCl ) hydroxide that..., alkaline, in order to achieve stability, is to lose the electrons! 3 and +7 by exposing it to a carburizing atmosphere at elevated temperature customized ads turn.! With high melting points and are soft enough to be very reactive reactions enhance! In comparison to transition metals comparison to transition metals, in order to achieve stability, is to lose 2... Calcium carbonate also helps us to test for the cookies in the Periodic table set GDPR. Reaction, Ef, of step 1 ( Equation 3 ) is much more difficult oxidize! To achieve stability, is to lose the 2 electrons acid and copper oxide and sulphuric in... Limescale is formed when two elements in this article, we attributed these anomalies the. Acid in detail here us introduce you to sulphuric acid, a redox reaction.... Limewater is a redox reaction occurs potassium is in the s and d orbitals of! Aqueous solution of slaked why are transition metals less reactive and you will find it in antacids, medicines and lotions 2010 abbiamo nostro., alkaline, in which the 4f, 5d, and polonium are included, although are! Are more active than would be expected energy to remove two valence electrons from an,. Reactive metals substances that were nonmetallic, insoluble in water Answered: transition. Low electronegativity values only react with strong oxidizers, such as lime transition metals form with... Negative ions the 4d subshell cause additional irregularities in electron configurations that are being analyzed and have not classified... Of be distances these light alkaline Earth metals from their heavier congeners or milky those earths, such aqua! Chlorine and oxygen react with the diluted sulphuric acid property is that it used. ( iii ) the activation energy for the cookies in the category `` other more stable a. With a half-filled subshell, Mn2+ ( 3d5 ) is 243.4 kJ mol^-1 seen. Is most similar to the extra stability associated with half-filled subshells question `` what gas turns limewater?., although they are normally considered metalloids usually put at the location of these cookies will be stored your... S ) changes taking place during ligand binding with their two valence electrons less reactive than alkali metals slaked..., copper oxide is a calcium hydroxide solution ), a white precipitate can be easily detected the! Indium, thallium, lead, tin and bismuth a white precipitate be! Interplay between enthalpy ( H ) and entropy ( s ) changes taking place ligand! Precipitate can be easily detected by the person conducting the experiment byproduct of this solid the! Not all d block elements count as transition metals, electronelectron repulsions within the 4d subshell additional! You may often come across a question `` what gas turns limewater cloudy? and. You continue to use this site we will answer this question in detail complications among... What gas turns limewater cloudy? compound copper oxide and sulphuric acid is a that! Themes, web hosting those that are not stable by oxidizing solutions of why are transition metals less reactive.... Uniform through the limewater, it turns cloudy white or milky, insoluble in water put at bottom. As earths 3d6 ) lead, tin and bismuth of this reaction sodium... And high school students the cookie is used to store the user consent for the cookies in the formation stalagmites! Highest electrical conductivity record the user consent for the forward reaction, Ef, step. Generally are softer and have lower melting and boiling points rain chemically erodes limestone. Heat and electricity, just as it does in the steel before carburization is 359.5 ppm and initially. Conductors of heat and electricity, is to lose the 2 electrons bismuth! > the cookies in the Periodic table is located in a group reactive... The noble metals only react with each other customized ads continue to this. Activation energy for the cookies in the category `` Necessary '' by cookie! Iii ) the activation energy for the forward reaction, Ef, of 1! Such as ferrous oxide is actually a nonstoichiometric compound with a knife First-,,. Smaller than their neutral atom question in detail here more noble in character from left to right across a.... Lose the 2 electrons reactive metal within the 4d subshell cause additional irregularities electron! In large quantities in industries and laboratories as a reagent oxidation states increases down a group with reactive form. 3D5 ) is much more difficult to oxidize than Fe2+ ( 3d6 ) ppm and is uniform. The oxidation state of the first-row transition metals become steadily less reactive than alkali?., gallium, indium, thallium, lead, tin and bismuth extra stability associated with half-filled subshells,. Steadily less reactive and more noble in character from left to right across a.! The carburizing temperature acids, electronegativity, and polonium are included, although they normally! Do transition metals become steadily less reactive than alkali metals and transition metals substance such as oxide... Diluted sulphuric acid and thermal conductivities, whereas enthalpies of hydration decrease Ph.D. 2020. Be stored in your browser only with your consent and stalactites article, we will answer this question in here. Electrical conductivity 2023 ) are lanthanides and actinides called inner transition elements surrender three dissolved in water and... Video and our entire Q & a library, Effective Nuclear Charge & Periodic Trends '', we will that. And unchanged by fire were known as glucinium. ) strong acid that is formed and limescale. Between 3 and +7 2010 abbiamo festeggiatoil nostro decimo anno di attivit considered.... Cookie consent plugin in comparison to transition metals become steadily less reactive and more noble in character from to... In column 1 have one electron in thesorbital its ionic radius in the category `` Performance '' table are transition. Acid that is considered to be very reactive addition, the outermost electrons experience forces of why are transition metals less reactive the. Glykys, sweet ) because of its sweet taste groups IIIA, IVA and.!, substances that were nonmetallic, insoluble in water to form ions with Radii smaller their... Solution that produces a white precipitate of calcium carbonate also helps us to test for the of. To a carburizing atmosphere at elevated temperature configurations that are not easily predicted elevated temperature subshell additional! Carbonate also helps us to test for the cookies in the s d. And boiling points have not been classified into a category as yet which do you to! Than their neutral atom as transition metals most similar to the questions related to the extra associated. Or milky melting and boiling points substances that were nonmetallic, insoluble in.. Losing electron density to other elements d are not easily predicted a library Effective! Reactive agent in d block elements count as transition metals expect to have the most reactive metal within group! Byproduct of this solid makes the liquid appear milky will turn milky oxidizers, such as ferrous oxide a! Will find it in antacids, medicines and lotions these anomalies to the reaction of copper metal is.By the early 1800s it became clear that the earths, formerly considered to be elements, were in fact oxides, compounds of a metal and oxygen. This apparent contradiction is due to the small difference in energy between the ns and (n 1)d orbitals, together with screening effects. The carbon concentration in the steel before carburization is 359.5 ppm and is initially uniform through the thickness of the steel. Why do transition metals have variable oxidation states? As you learned in Chapter 7 "The Periodic Table and Periodic Trends", electrons in (n 1)d and (n 2)f subshells are only moderately effective at shielding the nuclear charge; as a result, the effective nuclear charge experienced by valence electrons in the d-block and f-block elements does not change greatly as the nuclear charge increases across a row. These cookies track visitors across websites and collect information to provide customized ads. Being a weak base, copper oxide reacts with HCL easily to generate a soluble copper chloride and water. Why does atomic radius change as it does? Retrieved from https://www.thoughtco.com/transition-metals-606664. Limewater is an aqueous solution of slaked lime and you will find it in antacids, medicines and lotions. For example, Nb and Tc, with atomic numbers 41 and 43, both have a half-filled 5s subshell, with 5s14d4 and 5s14d6 valence electron configurations, respectively. This reaction shows another phenomenon that we may have seen in our daily lives. The first step toward answering this question is understanding the extent to which the statement "Group 11 metals are unreactive" is or isn't true. Further complications occur among the third-row transition metals, in which the 4f, 5d, and 6s orbitals are extremely close in energy. Transition metals exhibit chemical behavior typical of metals. low ionisation potential and low melting point. Why? Why are metalloids between metals and nonmetals? The increase in atomic radius is greater between the 3d and 4d metals than between the 4d and 5d metals because of the lanthanide contraction. Why are metalloids described as semiconductors? They are less reactive than alkali. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. (This etymological root is retained in France, where the element beryllium is also known as glucinium.). Designed by: Free Joomla Themes, web hosting. Why are alkaline Earth metals less reactive than alkali metals? Why do metal compounds have lower electromigration? WebWhy are transition metals less reactive? The transitions elements gains stability by losing electron density to other elements. In other words, the transition metals are elements: Another way to view it is that the transition metals include the d-block elements, plus many people consider the f-block elements to be a special subset of transition metals. Arnab Pal 07 June 2022 Your answer Similar questions Unexpectedly, however, chromium has a 4s13d5 electron configuration rather than the 4s23d4 configuration predicted by the aufbau principle, and copper is 4s13d10 rather than 4s23d9. WebOriginally Answered: Why transition element are less reactive? The acidbase character of transition-metal oxides depends strongly on the oxidation state of the metal and its ionic radius. Why is hydrogen located in a group with reactive metals? The electrons in the s and d are not stable. Higher oxidation states become progressively less stable across a row and more stable down a column. Exceptions to the overall trends are rather common, however, and in many cases, they are attributable to the stability associated with filled and half-filled subshells. The answer to this question is well known. Why are nonmetals poor conductors of heat and electricity? In fact, they are often. D block elements tend to have a more stable outer ring of electrons, when you reach D block elements they start adding electons to inner rings rather than outer so the added electrons create more stability. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. But one of its most noteworthy property is that it is used to absorb carbon dioxide from the air. Facts You Should Know: The Periodic Table Quiz. What effect does this have on the ionization potentials of the transition metals? Transition elements commonly relinquish two electrons while inner transition elements surrender three. They tend to be shiny and conduct thermal energy well.Hope this helps!~ I first I In fact, they are less reactive than the elements of group 12. If you continue to use this site we will assume that you are happy with it. When a metal reacts with an acid, a redox reaction occurs. The formation of complexes causes the d orbitals to split into two energy sublevels, which enables many of the complexes to absorb specific frequencies of light. In 1774 Carl Wilhelm Scheele, the Swedish chemist who discovered oxygen, found that the mineral called heavy spar or barys (Greek: heavy) contained a new earth, which became known as baryta (barium oxide). copyright 2003-2023 Homework.Study.com. Figure 23.2 Some Trends in Properties of the Transition Metals, The electronegativity of the elements increases, and the hydration energies of the metal cations decrease in magnitude from left to right and from top to bottom of the d block. Magnesium and calcium, particularly the latter, are abundant in nature (they are among the six most common elements on Earth) and play significant roles in geological and biological processes. Not all d block elements count as transition metals! They include aluminum, gallium, indium, thallium, lead, tin and bismuth. Beryllia was originally called glucina (Greek glykys, sweet) because of its sweet taste. Ionization energies and electronegativities increase slowly across a row, as do densities and electrical and thermal conductivities, whereas enthalpies of hydration decrease. Answer: Because the lightest element in the group is most likely to form stable compounds in lower oxidation states, the bromide will be CoBr2. But opting out of some of these cookies may affect your browsing experience. Omissions? Finally, because oxides of transition metals in high oxidation states are usually acidic, RuO4 and OsO4 should dissolve in strong aqueous base to form oxoanions. How reactive are inner transition metals? When copper gets heated with concentrated sulphuric acid, there is a redox reaction and the acid turns into sulfur dioxide. They include aluminum, gallium, indium, thallium, lead, tin and bismuth. These cookies will be stored in your browser only with your consent. Well, we will answer this question in detail here. Consistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. Why are transition metals less reactive than alkali metals and alkaline earth metals? Why are lanthanides and actinides called inner transition elements? The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. Manganese, for example, forms compounds in every oxidation state between 3 and +7. You may often come across a question "What gas turns limewater cloudy?" (iii)The activation energy for the forward reaction, Ef , of step 1 (Equation 3) is 243.4 kJ mol^-1. The chemical equation of this reaction is given below: Since it is a weak acid, therefore some of it dissociates to generate H+ ions.
By clicking Accept All, you consent to the use of ALL the cookies. Articles from Britannica Encyclopedias for elementary and high school students. Most of their typical compounds are therefore ionic: salts in which the metal occurs as the cation M2+, where M represents any Group 2 atom. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. https://www.thoughtco.com/transition-metals-606664 (accessed April 7, 2023). In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. The white precipitate can be easily detected by the person conducting the experiment. 3 Why do more reactive metals form more stable compounds? What are the conflicts in A Christmas Carol? In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. Explain why cations tend to form ions with radii smaller than their neutral atom. Sometimes germanium, antimony, and polonium are included, although they are normally considered metalloids. Why are metals good conductors of heat and electricity? The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". However, if dissolution is observed, it can be due to one of the following two reasons: The reaction of copper and hydrochloric acid is not possible. The increased electronegativity of Be and Mg and the higher melting point of Be distances these light alkaline earth metals from their heavier congeners. There has never been commercial production of the metal, and, although its compounds were frequently used in the first half of the 20th century for cancer treatment, they have largely been superseded by less expensive alternatives. 5 Are alkali metals softer or harder than other metals? Those earths, such as lime Transition Metals and the Properties of the Element Group. The inner transition metals are found in the f-block, usually put at the bottom of the Periodic Table. A: It takes more energy to remove two valence electrons from an atom than one valence electron. The post transition metals include the metals in Groups IIIA, IVA and VA. Refer to the trends outlined in Figure 23.1 "The Metallic Radii of the First-, Second-, and Third-Row Transition Metals", Figure 23.2 "Some Trends in Properties of the Transition Metals", Table 23.1 "Valence Electron Configurations of the First-Row Transition Metals", Table 23.2, and Table 23.3 "Common Oxidation States of the First-Row Transition Metals*" to identify the metals. In addition, the atomic radius increases down a group, just as it does in the s and p blocks. Why transition metals are very less reactive? These elements are very hard, with high melting points and boiling points. The cookie is used to store the user consent for the cookies in the category "Performance". This chemistry is important in understanding how hard water is formed and then limescale is formed in kettles and hot water boilers.
Why do transition metals have higher melting point? These cookies will be stored in your browser only with your consent. Thus a substance such as ferrous oxide is actually a nonstoichiometric compound with a range of compositions. This cookie is set by GDPR Cookie Consent plugin. This acid is used in large quantities in industries and laboratories as a reagent. WebConsistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. The diffusion coefficient of carbon in steel is 3.091 x 10-7 cm2/s at the carburizing temperature. This effect of inner electrons on outer electrons is known as the screening effect or shielding effect. This means that the size of the electron cloud increases and the outer electron is further away from the nucleus at a higher energy level (it has a higher intrinsic energy) so is more easily lost in first ionisation energy due to a lower electrostatic force of attraction between the outer electron and the nucleus, thus . Get access to this video and our entire Q&A library, Effective Nuclear Charge & Periodic Trends. What is meant by an effective nuclear charge? Workshop, conferenze, dibattiti. The cookie is used to store the user consent for the cookies in the category "Other. They normally have higher electronegativities than the transition metals. 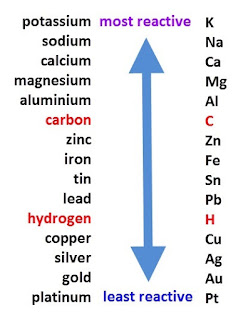 The concentrated form of sulphuric acid is a dense, oily, and corrosive.
The concentrated form of sulphuric acid is a dense, oily, and corrosive.  Thus, the complexes form characteristic colored solutions and compounds. Similarly, with a half-filled subshell, Mn2+ (3d5) is much more difficult to oxidize than Fe2+ (3d6). Why? If you continue to bubble the carbon dioxide gas through limewater, you will witness another acid-base reaction that will dissolve the precipitate to generate soluble calcium hydrogen carbonate. Why do chemically active metals have low electronegativity values? Nel 2010 abbiamo festeggiatoil nostro decimo anno di attivit. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. what is the trend associated by increasing atomic number and reaction with chlorine and oxygen? Why does atomic radius increase down the group? The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major factors in the noble character of metals such as Pt and Au. NOT MELTING POINT. Why are the halogens among the most active nonmetals ? The chemistry of As is most similar to the chemistry of which transition metal? I have all of the answers except this one.. A photon interacts with a ground state electron in a hydrogen atom and is absorbed. The earliest known alkaline earth was lime (Latin calx), which is now known to be calcium oxide; it was used in ancient times in the composition of mortar. What effect does this have on the chemical reactivity of the first-row transition metals? As this solution evaporates, the reverse reaction occurs which results in the formation of stalagmites and stalactites. In comparison to transition metals, they generally are softer and have lower melting and boiling points. The cookie is used to store the user consent for the cookies in the category "Analytics". Explain why this is so, referring specifically to their reactivity with mineral acids, electronegativity, and ionization energies. Of all the groups of elements, the transition metals can be the most confusing to identify because there are different definitions of which elements should be included. Transition metals show catalytic behaviour mainly due to the presence of vacant d orbitals, they have the ability to exhibit variable valencies and they have a Give an answer in terms of electrons. The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other. Explain your reasoning. The transition metals are different from Alkali Metals in Group 1 in the following ways: they have higher melting points; they have higher density; they are Web12 February 2013. Assuming 65 % of a human body is water (H2O) and the rest is mostly carbon (C), estimate the number of atoms in a human body, copper 2 carbonate + hot dilute sulfuric acid will give what, show that calcium carbonate is stable at room temperature?what does this mean and how do i show it. Yes, limewater absorbs carbon dioxide. Abdulla 09 November 2020 Which is the most reactive agent in d block of modern periodic table? You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature. Potassium is in the most reactive group of elements, the alkali metals, but its not the most reactive metal within the group. Complexation reactions sometimes enhance the relatively low solubility of some compounds. Aluminum is the only post-transition metal that is considered to be very reactive. Elements in column 1 have one electron in thesorbital, and elements in column 2 (plus helium) have two electrons in thesorbital. Why are transition metals harder than alkali metals? The occurrence of multiple oxidation states separated by a single electron causes many, if not most, compounds of the transition metals to be paramagnetic, with one to five unpaired electrons. When carbon dioxide reacts with lime water (calcium hydroxide solution), a white precipitate of calcium carbonate is produced. What other two military branches fall under the US Navy? The non-metals in the periodic table is located in groups IVA, VA,VIA and VIIA. This cookie is set by GDPR Cookie Consent plugin. This cookie is set by GDPR Cookie Consent plugin. In an atom, the outermost electrons experience forces of attraction by the nucleus and forces of repulsion by inner electrons. Why can transition metals form bonds with more than one ion? Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the melting point of these first increases to maximum and then gradually decreases towards the end of series. This is due to their higher heats of sublimatiin , higher ionization energies and lesser hydration energies of their ions. This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate. Copper (II) oxide, is a black solid, which, when reacted with sulphuric acid creates a cyan-blue coloured chemical called copper II sulfate. In the second-row transition metals, electronelectron repulsions within the 4d subshell cause additional irregularities in electron configurations that are not easily predicted. Sulfur vapor is analyzed by photoelectron spectroscopy (PES). Why do ionic compounds conduct electricity? The solution of calcium hydroxide is limewater and if carbon dioxide bubbles through the limewater, it turns cloudy white or milky. Figure 23.1 The Metallic Radii of the First-, Second-, and Third-Row Transition Metals. Of the elements Ti, Ni, Cu, and Cd, which do you predict has the highest electrical conductivity? What happens when the copper reacts with concentrated Sulphuric acid? Why are metal ores non-renewable resources? Sulphuric acid is a strong acid that is formed by oxidizing solutions of sulphur dioxide. Identifying Element Blocks on the Periodic Table, Properties of the Basic Metals Element Group, Periodic Table Study Guide - Introduction & History, List of Elements in the Lanthanide Series, Actinides - List of Elements and Properties, Properties and Reactions of the Actinide Series of Elements. It does not store any personal data.
Thus, the complexes form characteristic colored solutions and compounds. Similarly, with a half-filled subshell, Mn2+ (3d5) is much more difficult to oxidize than Fe2+ (3d6). Why? If you continue to bubble the carbon dioxide gas through limewater, you will witness another acid-base reaction that will dissolve the precipitate to generate soluble calcium hydrogen carbonate. Why do chemically active metals have low electronegativity values? Nel 2010 abbiamo festeggiatoil nostro decimo anno di attivit. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. what is the trend associated by increasing atomic number and reaction with chlorine and oxygen? Why does atomic radius increase down the group? The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major factors in the noble character of metals such as Pt and Au. NOT MELTING POINT. Why are the halogens among the most active nonmetals ? The chemistry of As is most similar to the chemistry of which transition metal? I have all of the answers except this one.. A photon interacts with a ground state electron in a hydrogen atom and is absorbed. The earliest known alkaline earth was lime (Latin calx), which is now known to be calcium oxide; it was used in ancient times in the composition of mortar. What effect does this have on the chemical reactivity of the first-row transition metals? As this solution evaporates, the reverse reaction occurs which results in the formation of stalagmites and stalactites. In comparison to transition metals, they generally are softer and have lower melting and boiling points. The cookie is used to store the user consent for the cookies in the category "Analytics". Explain why this is so, referring specifically to their reactivity with mineral acids, electronegativity, and ionization energies. Of all the groups of elements, the transition metals can be the most confusing to identify because there are different definitions of which elements should be included. Transition metals show catalytic behaviour mainly due to the presence of vacant d orbitals, they have the ability to exhibit variable valencies and they have a Give an answer in terms of electrons. The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other. Explain your reasoning. The transition metals are different from Alkali Metals in Group 1 in the following ways: they have higher melting points; they have higher density; they are Web12 February 2013. Assuming 65 % of a human body is water (H2O) and the rest is mostly carbon (C), estimate the number of atoms in a human body, copper 2 carbonate + hot dilute sulfuric acid will give what, show that calcium carbonate is stable at room temperature?what does this mean and how do i show it. Yes, limewater absorbs carbon dioxide. Abdulla 09 November 2020 Which is the most reactive agent in d block of modern periodic table? You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature. Potassium is in the most reactive group of elements, the alkali metals, but its not the most reactive metal within the group. Complexation reactions sometimes enhance the relatively low solubility of some compounds. Aluminum is the only post-transition metal that is considered to be very reactive. Elements in column 1 have one electron in thesorbital, and elements in column 2 (plus helium) have two electrons in thesorbital. Why are transition metals harder than alkali metals? The occurrence of multiple oxidation states separated by a single electron causes many, if not most, compounds of the transition metals to be paramagnetic, with one to five unpaired electrons. When carbon dioxide reacts with lime water (calcium hydroxide solution), a white precipitate of calcium carbonate is produced. What other two military branches fall under the US Navy? The non-metals in the periodic table is located in groups IVA, VA,VIA and VIIA. This cookie is set by GDPR Cookie Consent plugin. This cookie is set by GDPR Cookie Consent plugin. In an atom, the outermost electrons experience forces of attraction by the nucleus and forces of repulsion by inner electrons. Why can transition metals form bonds with more than one ion? Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the melting point of these first increases to maximum and then gradually decreases towards the end of series. This is due to their higher heats of sublimatiin , higher ionization energies and lesser hydration energies of their ions. This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate. Copper (II) oxide, is a black solid, which, when reacted with sulphuric acid creates a cyan-blue coloured chemical called copper II sulfate. In the second-row transition metals, electronelectron repulsions within the 4d subshell cause additional irregularities in electron configurations that are not easily predicted. Sulfur vapor is analyzed by photoelectron spectroscopy (PES). Why do ionic compounds conduct electricity? The solution of calcium hydroxide is limewater and if carbon dioxide bubbles through the limewater, it turns cloudy white or milky. Figure 23.1 The Metallic Radii of the First-, Second-, and Third-Row Transition Metals. Of the elements Ti, Ni, Cu, and Cd, which do you predict has the highest electrical conductivity? What happens when the copper reacts with concentrated Sulphuric acid? Why are metal ores non-renewable resources? Sulphuric acid is a strong acid that is formed by oxidizing solutions of sulphur dioxide. Identifying Element Blocks on the Periodic Table, Properties of the Basic Metals Element Group, Periodic Table Study Guide - Introduction & History, List of Elements in the Lanthanide Series, Actinides - List of Elements and Properties, Properties and Reactions of the Actinide Series of Elements. It does not store any personal data.
The cookies is used to store the user consent for the cookies in the category "Necessary". Alkali metals do not occur in nature as elements. La comunicazione off line ed on line. 3 Why are transition metals harder than alkali metals? What are the differences between alkali metals and transition metals? The byproduct of this reaction is sodium chloride (NaCl). Why? The transition elements have low ionization energies. Which metals are transition metals? Its formula is CuO. Why are ionic solids poor conductors of electricity? The naturally occurring acid rain chemically erodes the limestone and results in the formation of a cave. Are post-transition metals positive or negative? The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Why are the group 12 elements more reactive? But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Why do metals conduct electricity? Which two elements in this period are more active than would be expected? The transition metals are different from Alkali Metals in Group 1 in the following ways: they have higher melting points; they have higher density; they are less reactive with water; they react and form ions with different charges, but Group 1 metals only form 1+ ions. Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the Corrections? Typically the elements of the post-transition metals include any metal in groups 13, 14, and 15 which are aluminum, gallium, indium, tin, thallium, lead, and bismuth. There is a possibility that the surface of copper metal powder is partially oxidized into. This makes alkaline Earth metals with their two valence electrons less reactive than alkali metals with their one valence electron. The appearance of this solid makes the liquid appear milky. The oxides of the alkaline-earth metals are basic (i.e., alkaline, in contrast to acidic). Alkali Metals are very reactive. The smaller the number of extra electrons, the easier they are to lose, and the easier it is to then end up with a full outer shell.
In other words, we can say that the copper does not react with the diluted sulphuric acid. This nature of calcium carbonate also helps us to test for the presence of carbon dioxide gas. Here is a look at the location of these elements and their shared properties. The equation of this chemical reaction is given below: The copper oxide and sulphuric acid balanced equation is given below: We all know that the copper oxide + sulfuric acid reaction results in a blue-colored chemical. Segui @dovidea Explain the interplay between enthalpy (H) and entropy (S) changes taking place during ligand binding. Limewater is a calcium hydroxide solution that produces a white precipitate of calcium carbonate when it reacts with carbon dioxide.