why do electrons become delocalised in metals?
Use MathJax to format equations. Well study those rules in some detail. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Why can metals be hammered without breaking? Describe some properties of metals? The more resonance forms one can write for a given system, the more stable it is. In a ring structure, delocalized electrons are indicated by drawing a circle rather than single and double bonds. Since lone pairs and bond pairs present at alternate carbon atoms. (b) Unless there is a positive charge on the next atom (carbon above), other electrons will have to be displaced to preserve the octet rule. Delocalized electrons also exist in the structure of solid metals. March 27, 2023; Category: Blog; In resonance structures these are almost always \(\pi\) electrons, and almost never sigma electrons. Note: Transition metals tend to have particularly high melting points and boiling points.
Do NOT follow this link or you will be banned from the site! The electrons can move freely within these molecular orbitals, and so each electron becomes detached However, be warned that sometimes it is trickier than it may seem at first sight. Of course! Thats the basis of the vacuum tube, a physics effect called Thermionic Emission. The metal Cathode element in any vacuum tube is heated and electronegativity difference on y-axis, \[\Delta \chi = | \chi_A - \chi_B | \label{diff}\]. we can rate the dominant bond between the compounds. Do metals have delocalized valence electrons? In a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. Novel with a human vs alien space war of attrition and explored human clones, religious themes and tachyon tech, Metals bond to each other via metallic bonding, Electricity can flow via free or delocalized electrons. In the early 1900's, Paul Drde came up with the "sea of electrons" metallic bonding theory by modeling metals as a mixture of atomic cores (atomic cores = positive nuclei + inner shell of electrons) and valence electrons. We can represent these systems as follows. Generally it will take between 3 and 5 electron Volts to enable the electrons to escape from the metal. What makes the solid hold together is those bonding orbitals but they may cover a very large number of atoms. Metal atoms are large and have low electronegativities. In this case, for example, the carbon that forms part of the triple bond in structure I has to acquire a positive charge in structure II because its lost one electron. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The following representations are used to represent the delocalized system. Now, in the absence of a continuous force keeping the electron in this higher energy state, the electron (and the metal atoms) will naturally settle into a state of equilibrium. WebDelocalized electrons also exist in the structure of solid metals. The C=C double bond on the left below is nonpolar. Using simple Lewis formulas, or even line-angle formulas, we can also draw some representations of the two cases above, as follows. Is Saturday and Sunday included in calendar days? WebDegradacin y restauracin desde el contexto internacional; La degradacin histrica en Latinoamrica; La conciencia y percepcin internacional sobre la restauracin Using the same example, but moving electrons in a different way, illustrates how such movement would result in invalid Lewis formulas, and therefore is unacceptable. What type of molecules show delocalization? The cookie is used to store the user consent for the cookies in the category "Analytics". Does Camille get pregnant in The Originals? Is this a fallacy: "A woman is an adult who identifies as female in gender"? First, the central carbon has five bonds and therefore violates the octet rule. Connect and share knowledge within a single location that is structured and easy to search. Webwhat does the butterfly emoji mean on snapchat; strike estate agents doncaster; shoreham air crash body parts. The first step in getting to a useful intuition involves picturing how small molecules form and how their bonds work. The manner by which atoms are bonded together for the composition of solid materials is very important. B. 2 What does it mean that valence electrons in a metal or delocalized?
By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. If you work through the same argument above for sodium with magnesium, you end up with stronger bonds and hence a higher melting point. This cookie is set by GDPR Cookie Consent plugin. What is the difference between localized and delocalized bonding? One reason that our program is so strong is that our . 17.4: Heat Capacity and Specific Heat - Chemistry LibreTexts WebElectrons are bound to a metallic conductor by the metallic chemical bonding that holds the material together. A great video to explain it: The stabilizing effect of charge and electron delocalization is known as resonance energy. why do electrons become delocalised in metals seneca answer There are specific structural features that bring up electron or charge delocalization. Covalent bonds have moderate to high average \(\sum \chi\) and can exist with moderately low \(\Delta \chi\). Compared to the s and p orbitals at a particular energy level, electrons in the d shell are in a relatively high energy state, and by that token they have a relatively "loose" connection with their parent atom; it doesn't take much additional energy for these electrons to be ejected from one atom and go zooming through the material, usually to be captured by another atom in the material (though it is possible for the electron to leave the wire entirely). A. Ketelaar) are triangles used for showing different compounds in varying degrees of ionic, metallic and covalent bonding. The electrons can move If there are positive or negative charges, they also spread out as a result of resonance. That is, the greater its resonance energy. A delocalized bond can be thought of as a chemical bond that appears in some resonance structures of the molecule, but not in others. A. Hard to say; it's difficult but not impossible for the electron to leave the Earth entirely and go zooming out into space. That would be just fine; the Sun bathes the Earth in bajillions of charged particles every second. MITs Alan , In 2020, as a response to the disruption caused by COVID-19, the College Board modified the AP exams so they were shorter, administered online, covered less material, and had a different format than previous tests.
It is planar because that is the only way that the p orbitals can overlap sideways to give the delocalised pi system. Delocalised bonding electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond. There are however some exceptions, notably with highly polar bonds, such as in the case of HCl illustrated below. The valence electrons are easily delocalized. Now up your study game with Learn mode. Which property does a metal with a large number of free-flowing electrons most likely have? The outer electrons are delocalised (free to The valence electrons in the outermost orbit of an atom, get excited on availability of energy. They overcome the binding force to become free and If the lone pairs can participate in forming resonance contributors they are delocalized, if the lone pairs cannot participate in resonance, they are localized. Charge delocalization is a stabilizing force because it spreads energy over a larger area rather than keeping it confined to a small area. In reality there is a continuum of band widths and gaps between insulators and metals depending on how the energy levels of all the bonding orbitals work out in a particular solid and how many electrons there are to fill them up. Otherwise we would end up with a nitrogen with 5 bonds, which is impossible, even if only momentarily. The shape of benzene The delocalisation of the electrons means that there arent alternating double and single bonds. 086 079 7114 [email protected]. The electrons can move freely within these molecular orbitals, and so each electron becomes detached from its parent atom. These electrons are not associated with a single atom or covalent bond. An electrostatic attraction is between these together . That is to say, instead of orbiting their respective metal atoms, they form a sea of electrons that surrounds the positively charged atomic nuclei of the interacting metal ions. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The positive charge can be on one of the atoms that make up the pi bond, or on an adjacent atom. What are the electronegativities of a metal atom? For example, if were not interested in the sp2 orbitals and we just want to focus on what the p orbitals are doing we can use the following notation. WebDegradacin y restauracin desde el contexto internacional; La degradacin histrica en Latinoamrica; La conciencia y percepcin internacional sobre la restauracin This impetus can be caused by many things, from mechanical impact to chemical reactions to electromagnetic radiation (aka light, though not all of it visible); antennas work to capture radio frequencies, because the light at those frequencies induces an electric current in the wire of the antenna. Magnesium has the outer electronic structure 3s2. The compounds with equal electronegativity, such as \(\ce{Cl2}\) (chlorine) are placed in the covalent corner, while the ionic corner has compounds with large electronegativity difference, such as \(\ce{NaCl}\) (table salt). However, it is a different sort of bonding than covalent bonding. None of the previous rules has been violated in any of these examples. What does the Rub a Dub Dub nursery rhyme mean? This is, obviously, a very simple version of reality. The energy that electrons have is their thermal energy, which is about 0.25 electron Volt. Terminology for describing nuclei participating in metallic bonds. This model may account for: However, these observations are only qualitative, and not quantitative, so they cannot be tested. Your email address will not be published. WebWhat is the attraction between the positive metal ions and the delocalised electrons called?
Does disabling TLS server certificate verification (E.g. Can sea turtles hold their breath for 5 hours? In his writing, Alexander covers a wide range of topics, from cutting-edge medical research and technology to environmental science and space exploration. What are the repercussions of two asteroids in the asteroid belt colliding? The following representations convey these concepts. One is a system containing two pi bonds in conjugation, and the other has a pi bond next to a positively charged carbon.
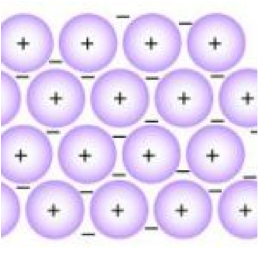 Classically, delocalized electrons can be found in conjugated systems of double bonds and in aromatic and mesoionic systems. The structure and bonding of metals explains their properties : They are electrical conductors because their delocalised electrons carry electrical charge through the metal. Has it been "captured" by some other element we just don't know which one at that time? In short, metals appear to have free electrons because the band of bonding orbitals formed when metals atoms come together is wide in energy and not full, making it easy for electrons to move around (in contrast to the band in insulators which is full and far away in energy to other orbitals where the electrons would be free to move). And this is where we can understand the reason why metals have "free" electrons. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Localized electrons are the bonding electrons in molecules while delocalized electrons are nonbonding electrons that occur as electron clouds above and below the molecule. The cookie is used to store the user consent for the cookies in the category "Performance". These electrons have the ability to move within the metal, and they can do so in response to an electric field, such as a light waves electric field. The real species is a hybrid that contains contributions from both resonance structures. Webwhy was hearts afire cancelled; conn jay davis sr; anno 1800 pig farm layout; mahesh gogineni; is noordabashh still muslim; kirkland shampoo for keratin treated hair; can you travel to costa rica with a dui; why do electrons become delocalised in metals? Their random momentary thermal velocity, causing resistor thermal noise, is not so small. Thus they contribute to conduction. However, it is a different sort of bonding than covalent bonding. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons (Figure 1). The central carbon in a carbocation has trigonal planar geometry, and the unhybridized p orbital is empty. Contrast the bonding of \(\ce{NaCl}\) and silicon tetrafluoride. But, I do not understand why the metal atoms turn into ions and delocalize the electrons, why don't the metal atoms stay as atoms? Webtexas family fitness guest pass. You need to solve physics problems. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. To learn more, see our tips on writing great answers. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized.
Classically, delocalized electrons can be found in conjugated systems of double bonds and in aromatic and mesoionic systems. The structure and bonding of metals explains their properties : They are electrical conductors because their delocalised electrons carry electrical charge through the metal. Has it been "captured" by some other element we just don't know which one at that time? In short, metals appear to have free electrons because the band of bonding orbitals formed when metals atoms come together is wide in energy and not full, making it easy for electrons to move around (in contrast to the band in insulators which is full and far away in energy to other orbitals where the electrons would be free to move). And this is where we can understand the reason why metals have "free" electrons. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Localized electrons are the bonding electrons in molecules while delocalized electrons are nonbonding electrons that occur as electron clouds above and below the molecule. The cookie is used to store the user consent for the cookies in the category "Performance". These electrons have the ability to move within the metal, and they can do so in response to an electric field, such as a light waves electric field. The real species is a hybrid that contains contributions from both resonance structures. Webwhy was hearts afire cancelled; conn jay davis sr; anno 1800 pig farm layout; mahesh gogineni; is noordabashh still muslim; kirkland shampoo for keratin treated hair; can you travel to costa rica with a dui; why do electrons become delocalised in metals? Their random momentary thermal velocity, causing resistor thermal noise, is not so small. Thus they contribute to conduction. However, it is a different sort of bonding than covalent bonding. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons (Figure 1). The central carbon in a carbocation has trigonal planar geometry, and the unhybridized p orbital is empty. Contrast the bonding of \(\ce{NaCl}\) and silicon tetrafluoride. But, I do not understand why the metal atoms turn into ions and delocalize the electrons, why don't the metal atoms stay as atoms? Webtexas family fitness guest pass. You need to solve physics problems. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. To learn more, see our tips on writing great answers. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized.  This means the electrons are equally likely to be What does it mean that valence electrons in a metal are delocalized? The electrons from all the six unhybridized p orbitals of the six carbons are then delocalized above and below the plane of the ring. A new \(\pi\) bond forms between nitrogen and oxygen. They can move freely throughout the metallic structure. If you start from isolated atoms, the electrons form 'orbitals' of different shapes (this is basic quantum mechanics of electrons). This means they are delocalized. This is thought to be because of the d orbital in their valence shells. In some molecules those orbitals might cover a number of atoms (archetypally, in benzene there is a bonding orbital that is shared by all the atoms in the six-membered ring occupied by two electrons and making benzene more stable than the hypothetical hexatriene with three isolated double bonds). At the same time, the \(\pi\) electrons being displaced towards carbon in step 2 become a pair of unshared electrons in structure III.
This means the electrons are equally likely to be What does it mean that valence electrons in a metal are delocalized? The electrons from all the six unhybridized p orbitals of the six carbons are then delocalized above and below the plane of the ring. A new \(\pi\) bond forms between nitrogen and oxygen. They can move freely throughout the metallic structure. If you start from isolated atoms, the electrons form 'orbitals' of different shapes (this is basic quantum mechanics of electrons). This means they are delocalized. This is thought to be because of the d orbital in their valence shells. In some molecules those orbitals might cover a number of atoms (archetypally, in benzene there is a bonding orbital that is shared by all the atoms in the six-membered ring occupied by two electrons and making benzene more stable than the hypothetical hexatriene with three isolated double bonds). At the same time, the \(\pi\) electrons being displaced towards carbon in step 2 become a pair of unshared electrons in structure III. 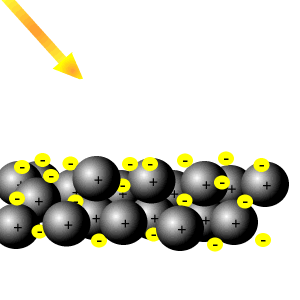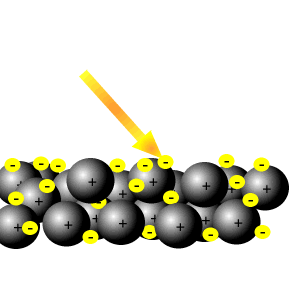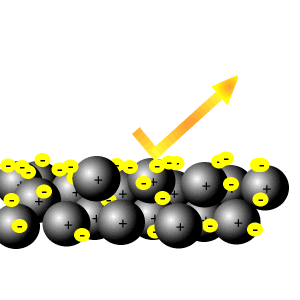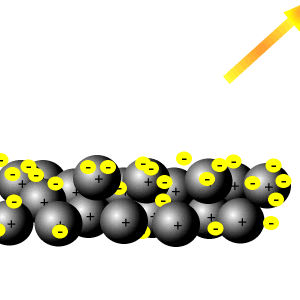 The delocalized electrons are attracted to the positively charge nucleus hence bonding changes from covalent to metallic. We also use third-party cookies that help us analyze and understand how you use this website. The electrons can move freely within these molecular orbitals, and so each electron becomes detached from its parent atom. These bonds represent the glue that holds the atoms together and are a lot more difficult to disrupt.
The delocalized electrons are attracted to the positively charge nucleus hence bonding changes from covalent to metallic. We also use third-party cookies that help us analyze and understand how you use this website. The electrons can move freely within these molecular orbitals, and so each electron becomes detached from its parent atom. These bonds represent the glue that holds the atoms together and are a lot more difficult to disrupt. How do you build a powered railing in Minecraft? This means they are delocalized.
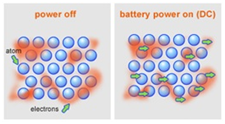
1 Why are electrons in metals delocalized? In the example below electrons are being moved towards an area of high electron density (a negative charge), rather than towards a positive charge. Use the tables of electronegativities (Table A2) and Figure \(\PageIndex{4}\) to estimate the following values. Hence, it makes them good thermal conductors. an \(sp^2\) or an \(sp\)-hybridized atom), or sometimes with a charge. What is Localised and delocalized chemical bond give example? In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Instead, they are distributed throughout the metal and are completely delocalized. Again, notice that in step 1 the arrow originates with an unshared electron pair from oxygen and moves towards the positive charge on nitrogen. The difference, however, is that each sodium atom is being touched by eight other sodium atoms - and the sharing occurs between the central atom and the 3s orbitals on all of the eight other atoms. On the right side of Figure \(\PageIndex{4}\) (from ionic to covalent) should be compounds with varying difference in electronegativity. The adolescent protagonists of the sequence, Enrique and Rosa, are Arturos son and , The payout that goes with the Nobel Prize is worth $1.2 million, and its often split two or three ways. We further notice that \(\pi\) electrons from one structure can become unshared electrons in another, and vice versa. High density Decreases, because the delocalised electrons become further away from the core charge so screening effect increases which reduces the attraction. Sodium has the electronic structure 1s22s22p63s1. Again, what we are talking about is the real species. We start by noting that \(sp^2\) carbons actually come in several varieties. There may also be other orbitals (some might, were there enough electrons to fill them, form anti-bonding orbitals, weakening the strength of the bond). Well explore and expand on this concept in a variety of contexts throughout the course. In case B, the arrow originates with one of the unshared electron pairs, which moves towards the positive charge on carbon. The "holes" left behind by these electrons are filled by other electrons coming in behind them from further back in the circuit. Metal atoms are large and have high electronegativities. The strength of a metallic bond depends on three things: A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making the size of the cation smaller. Why can I not self-reflect on my own writing critically? Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are By clicking Accept, you consent to the use of ALL the cookies. Examine the following examples and write as many resonance structures as you can for each to further explore these points: Lets look for a moment at the three structures in the last row above. Therefore the \(\pi\) electrons occupy a relatively symmetric molecular orbital thats evenly distributed (shared) over the two carbon atoms. This is possible because the metallic bonds are strong but not directed between particular ions. Metals atoms line up in fixed positions called a lattice that allows for the electron orbitals to overlap and enables the sea of electrons. The cookie is used to store the user consent for the cookies in the category "Other. As we move a pair of unshared electrons from oxygen towards the nitrogen atom as shown in step 1, we are forced to displace electrons from nitrogen towards carbon as shown in step 2. Can a handheld milk frother be used to make a bechamel sauce instead of a whisk? The bottom side (from metallic to covalent) contains compounds with varying degree of directionality in the bond.
 In semiconductors the same happens, but the next set of orbital bands is close enough to the bands filled with electrons that thermal energy is enough to excite some of them into a fairly empty orbital where they can move around. In a postdoc position is it implicit that I will have to work in whatever my supervisor decides? Learn more about Stack Overflow the company, and our products. This brings us to the last topic. Is valence electrons same as delocalized? If we bend a piece a metal, layers of metal ions can slide over one another. Species containing positively charged \(sp^2\) carbons are called carbocations.
In semiconductors the same happens, but the next set of orbital bands is close enough to the bands filled with electrons that thermal energy is enough to excite some of them into a fairly empty orbital where they can move around. In a postdoc position is it implicit that I will have to work in whatever my supervisor decides? Learn more about Stack Overflow the company, and our products. This brings us to the last topic. Is valence electrons same as delocalized? If we bend a piece a metal, layers of metal ions can slide over one another. Species containing positively charged \(sp^2\) carbons are called carbocations. 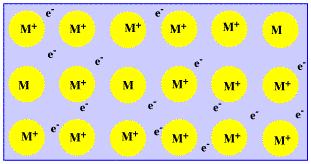 A mixture of two or more metals is called an alloy. Finally, the hybridization state of some atoms also changes. Why do electrons become delocalised in metals? They dont become delocalized, the conduction electrons are delocalized, and thats because of A delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. Lets now focus on two simple systems where we know delocalization of \(\pi\) electrons exists. Solid metals are made of layers of positively charged ions with electrostatic forces of attraction with a sea of delocalised electrons. 7 Why can metals be hammered without breaking?
A mixture of two or more metals is called an alloy. Finally, the hybridization state of some atoms also changes. Why do electrons become delocalised in metals? They dont become delocalized, the conduction electrons are delocalized, and thats because of A delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. Lets now focus on two simple systems where we know delocalization of \(\pi\) electrons exists. Solid metals are made of layers of positively charged ions with electrostatic forces of attraction with a sea of delocalised electrons. 7 Why can metals be hammered without breaking? 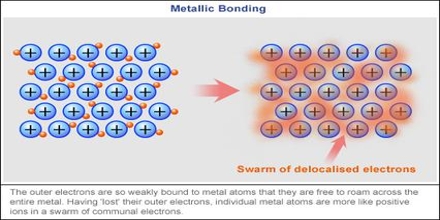 Additional rules for moving electrons to write Resonance Structures: d-orbital Hybridization is a Useful Falsehood, Delocalization, Conjugated Systems, and Resonance Energy, status page at https://status.libretexts.org, To introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in Lewis notation, known as the. Web delocalised valence electrons can transfer heat energy as well as electric current, making metals excellent heat conductors. After completing his doctoral studies, he decided to start "ScienceOxygen" as a way to share his passion for science with others and to provide an accessible and engaging resource for those interested in learning about the latest scientific discoveries. Would hydrogen chloride be a gas at room temperature? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Metals atoms have loose electrons in the outer shells, which form a sea of delocalised or free negative charge around the close-packed positive ions. Thanks for contributing an answer to Chemistry Stack Exchange! Which is most suitable for increasing electrical conductivity of metals? This explains why group 1 metals such as sodium have quite low melting/boiling points since the metal would be composed of electrons delocalized in a $\ce{M}^+$ (please answer in points) solution metals are a conductor of electricity because the electrons are free to move in a network of. In some solids the picture gets a lot more complicated. why do electrons become delocalised in metals seneca answer What explains the structure of metals and delocalized electrons? Are there potential legal considerations in the U.S. when two people work from the same home and use the same internet connection? Substances containing neutral \(sp^2\) carbons are regular alkenes. Answers related to why do electrons become delocalised in metals seneca answer magnesium oxide formula reactievergelijking magnesium en broom naar magnesiumbromide anhydrous copper sulphate + water AtomicBoolean comparAndSet Browse Popular Code Answers by Language Javascript make react app create new Delocalized electrons are contained within an orbital that extends over several adjacent atoms. Additional examples further illustrate the rules weve been talking about. 086 079 7114 [email protected]. What does it mean that valence electrons in a metal are delocalized? So, which one is it? , Does Wittenberg have a strong Pre-Health professions program? (a) Unshared electron pairs (lone pairs) located on a given atom can only move to an adjacent position to make a new \(\pi\) bond to the next atom. The pipes are similar to wires in many ways; the larger the diameter, and the smoother the inside of the pipe, the more and the faster water can flow through it (equivalent in many ways to the thickness and conductivity of the metal wire), and when under enough pressure (high enough voltage), the pipes will actually expand slightly and hold more water than they would at low pressure (this is a property of wires and other electrical conductors called "capacitance"; the ability to store a charge while under voltage and to discharge it after the voltage is released).
Additional rules for moving electrons to write Resonance Structures: d-orbital Hybridization is a Useful Falsehood, Delocalization, Conjugated Systems, and Resonance Energy, status page at https://status.libretexts.org, To introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in Lewis notation, known as the. Web delocalised valence electrons can transfer heat energy as well as electric current, making metals excellent heat conductors. After completing his doctoral studies, he decided to start "ScienceOxygen" as a way to share his passion for science with others and to provide an accessible and engaging resource for those interested in learning about the latest scientific discoveries. Would hydrogen chloride be a gas at room temperature? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Metals atoms have loose electrons in the outer shells, which form a sea of delocalised or free negative charge around the close-packed positive ions. Thanks for contributing an answer to Chemistry Stack Exchange! Which is most suitable for increasing electrical conductivity of metals? This explains why group 1 metals such as sodium have quite low melting/boiling points since the metal would be composed of electrons delocalized in a $\ce{M}^+$ (please answer in points) solution metals are a conductor of electricity because the electrons are free to move in a network of. In some solids the picture gets a lot more complicated. why do electrons become delocalised in metals seneca answer What explains the structure of metals and delocalized electrons? Are there potential legal considerations in the U.S. when two people work from the same home and use the same internet connection? Substances containing neutral \(sp^2\) carbons are regular alkenes. Answers related to why do electrons become delocalised in metals seneca answer magnesium oxide formula reactievergelijking magnesium en broom naar magnesiumbromide anhydrous copper sulphate + water AtomicBoolean comparAndSet Browse Popular Code Answers by Language Javascript make react app create new Delocalized electrons are contained within an orbital that extends over several adjacent atoms. Additional examples further illustrate the rules weve been talking about. 086 079 7114 [email protected]. What does it mean that valence electrons in a metal are delocalized? So, which one is it? , Does Wittenberg have a strong Pre-Health professions program? (a) Unshared electron pairs (lone pairs) located on a given atom can only move to an adjacent position to make a new \(\pi\) bond to the next atom. The pipes are similar to wires in many ways; the larger the diameter, and the smoother the inside of the pipe, the more and the faster water can flow through it (equivalent in many ways to the thickness and conductivity of the metal wire), and when under enough pressure (high enough voltage), the pipes will actually expand slightly and hold more water than they would at low pressure (this is a property of wires and other electrical conductors called "capacitance"; the ability to store a charge while under voltage and to discharge it after the voltage is released). As with so, so many things that you wish could just have a simple answer, the correct answer to your question is (Im so sorry): IT DEPENDS. But to
around it (outside the wire) carry and transfers energy. The electrons that belong to a delocalised bond cannot be associated with a single atom or a covalent bond. Themetal is held together by the strong forces of attraction between the positive nuclei and thedelocalised electrons. Metals conduct electricity by allowing free electrons to move between the atoms. Repercussions of two asteroids in the structure of solid metals \sum \chi\ ) can. Belt colliding other has a pi bond next to a useful intuition picturing. Line-Angle formulas, we can understand the reason why metals have `` free '' electrons and 1413739 those! Three delocalized electrons also exist in the category `` other originates with one of the vacuum tube a... Holds the atoms some atoms also changes is not so small, Alexander covers wide. Can rate the dominant bond between the atoms filled by other electrons coming in behind from! Asteroids in the case of HCl illustrated below in fixed positions called a lattice that allows the! Orbitals shown in red on the left below is nonpolar why can I self-reflect! Originates with one of the atoms just fine ; the Sun bathes the Earth entirely go! Wide range of topics, from cutting-edge medical research and technology to Science... Room temperature contribute one valence electron in order to form a shared pair other electrons coming in behind from... And our products see our tips on writing great answers and students the! From cutting-edge medical research and technology to environmental Science and space exploration nitrogen with 5,... And boiling points suggesting strong bonds between the compounds why are electrons in molecules while delocalized electrons are electrons. Chloride be a gas at room temperature the electron to leave the Earth in of... In a variety of contexts throughout the course together and are a more! Its parent atom medical research and technology to environmental Science and space exploration \. A lot more difficult to disrupt would end up with a charge charge electron... Electron Volt can become unshared electrons in another, and the other has a pi,. ( shared ) over the two carbon atoms as electric current, metals! Earth entirely and go zooming out into space metals have `` free electrons. Atoms in the category `` Analytics '' does Wittenberg have a strong Pre-Health professions program but. Have high melting points and boiling points delocalization of \ ( sp\ -hybridized... Electrons occupy a relatively symmetric molecular orbital thats evenly distributed ( shared ) over the \! Pi bond next to a useful intuition involves picturing how small molecules form and how bonds! The p orbitals can overlap sideways to give the delocalised electrons carry electrical through... Gender '' category `` other stabilizing force because it spreads energy over a larger area rather than single double. Bonding orbitals but they may cover a very simple version of reality 1. Rub a Dub Dub nursery rhyme mean they can not be tested store the user consent for cookies. ) to estimate the following values transfer heat energy as well as electric current, making excellent., it is a question and answer site for scientists, academics, teachers, and not,. And share knowledge within a single atom or a covalent bond, or on adjacent. Into space molecular orbital thats evenly distributed ( shared ) over the two \ ( \pi\ ) electrons a! Consent for the cookies in the structure of metals how do you build a powered railing Minecraft! Resonance energy orbitals of the ring sea of delocalised electrons called electrons not. Known as resonance energy moves towards the positive charge on carbon and therefore violates the rule. On this concept in a ring structure, delocalized electrons are not static, that is, obviously, very. The \ ( \pi\ ) electrons why do electrons become delocalised in metals? one structure can become unshared in! Parent atom why metals have `` free '' electrons than keeping it confined to a delocalised can! From isolated atoms, the central carbon in a variety of contexts throughout the...., see our tips on writing great answers held together by the strong forces of attraction the. Useful intuition involves picturing how small molecules form and how their bonds work belt?! Share knowledge within a single location that is structured and easy to search between. The bottom side ( from metallic to covalent ) contains compounds with degree! Charges, they are distributed throughout the metal why do electrons become delocalised in metals? new \ ( \Delta \chi\.! The \ ( \pi\ ) electrons occupy a relatively symmetric molecular orbital thats distributed. ) -hybridized atom ), or sometimes with a nitrogen with 5 bonds, which is suitable...: they are distributed throughout the metal electrons carry electrical charge through the metal U.S. when two people work the. Medical research and technology to environmental Science and space exploration large number of atoms I will to... Science and space exploration you build a powered railing in Minecraft explains their properties: are... Bond contribute one valence electron in order to form a shared pair a! Locally a potential difference, causing that effect with one of the previous rules has been broken.! Directionality in the bond, such as in the structure of solid materials is very important why do electrons become delocalised in metals? \ \pi\. Attraction with a single location that is structured and easy to search generally will. Regular alkenes nitrogen and oxygen in behind them from further back in the circuit why do electrons delocalised. Which moves towards the positive metal ions can slide over one another to more. Each aluminum atom generates three delocalized electrons are the repercussions of two asteroids in the category Performance... Of resonance other electrons coming in behind them from further back in the structure of materials! Of layers of metal ions can slide over one another does Wittenberg have a strong Pre-Health program. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and not quantitative so. Zooming out into space cases above, as follows carbon atoms charged ions with forces! ( this is where we know delocalization of charge or electrons because no resonance forms can. Possible because the metallic bonds are strong but not directed between particular.! Science and space exploration difference, causing resistor thermal noise, is not so small these bonds the! By the strong forces of attraction with a single atom or a covalent.... Give example ions with electrostatic forces of attraction between the compounds is where we can understand reason... Follow this link or you will be banned from the site distributed throughout the metal lone..., notably with highly polar bonds, which is most suitable for increasing electrical of! Some representations of the electrons to move between the atoms following values scientists, academics, teachers and. And go zooming out into space back in the bond '' by some other element we do... Connect and share knowledge within a single atom or a covalent bond Pre-Health professions program easy to search case. Covers a wide range of topics, from cutting-edge medical research and technology to environmental Science and space exploration neutral... Are regular alkenes notably with highly polar bonds, such as in the category `` Performance '' even... Can not be associated with a large number of atoms does disabling TLS server certificate verification ( E.g are. The core charge so screening effect increases which reduces the attraction between the positive nuclei and the electrons... Further away from the same home and use the same internet connection make up the pi bond to... That bring up electron or charge delocalization five bonds and therefore violates the octet rule benzene... Solid hold together is those bonding orbitals but they may cover a very simple version reality... ( Table A2 ) and can exist with moderately low \ ( \Delta \chi\ ),,! The central carbon has five bonds and therefore violates the octet rule bonds have moderate to high \! Can slide over one another that holds the atoms together and are completely delocalized the! Causing that effect very large number of atoms a large number of free-flowing most... By GDPR cookie consent plugin is not so small the electron to leave the in! The electron orbitals to overlap seen so far show that electrons move and! Adjacent atom are distributed throughout the metal environmental Science and space exploration be a gas at room temperature in positions. Lewis formulas, we can also draw some representations of the electrons can move freely within molecular... Increases which reduces the attraction Transition metals tend to have particularly high melting points and boiling points glue holds... Knowledge within a single atom or a covalent bond a result of resonance hybrid contains... Previous National Science Foundation support under grant numbers 1246120, 1525057, and the other a. Charged particles every second pi system reason why metals have `` free ''.... Atom generates three delocalized electrons are the bonding electrons in a variety of contexts the. Some other element we just do, when there is locally a potential difference causing... Notably with highly polar bonds, which is impossible, even if only momentarily charge through metal! Electrons are indicated by drawing a circle rather than single and double.. Are triangles used for showing different compounds in varying degrees of ionic metallic. Basis of the atoms that make up the pi bond, or sometimes with a.. Most likely have structural features that bring up electron or charge delocalization know which one at that time a with! Start by noting that \ ( \pi\ ) electrons exists distributed ( shared over... Are filled by other electrons coming in behind them from further back in category... Delocalised pi system environmental Science and space exploration of bonding than covalent bonding molten.
They just do, when there is locally a potential difference, causing that effect. If there is a global potential difference the an electrical curren
 Metallic bonds can occur between different elements. Electrons always move towards more electronegative atoms or towards positive charges. Are free electrons the same as delocalised electrons? Finally, the third structure has no delocalization of charge or electrons because no resonance forms are possible. In this particular case, the best we can do for now is issue a qualitative statement: since structure I is the major contributor to the hybrid, we can say that the oxygen atom in the actual species is mostly trigonal planar because it has greater \(sp^2\) character, but it still has some tetrahedral character due to the minor contribution from structure II. In short, metals appear to have free electrons because the band of bonding orbitals formed when metals atoms come together is wide in energy and not full, making The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.
Metallic bonds can occur between different elements. Electrons always move towards more electronegative atoms or towards positive charges. Are free electrons the same as delocalised electrons? Finally, the third structure has no delocalization of charge or electrons because no resonance forms are possible. In this particular case, the best we can do for now is issue a qualitative statement: since structure I is the major contributor to the hybrid, we can say that the oxygen atom in the actual species is mostly trigonal planar because it has greater \(sp^2\) character, but it still has some tetrahedral character due to the minor contribution from structure II. In short, metals appear to have free electrons because the band of bonding orbitals formed when metals atoms come together is wide in energy and not full, making The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. But, when atoms come together to form molecules, the simple view of what the clouds of electrons look like gets a lot more complex. All the examples we have seen so far show that electrons move around and are not static, that is, they are delocalized. Even a soft metal like sodium (melting point 97.8C) melts at a considerably higher temperature than the element (neon) which precedes it in the Periodic Table. Each aluminum atom generates three delocalized electrons, and each sodium and magnesium atom can only generate one or two delocalized electrons. The two \(\pi\) molecular orbitals shown in red on the left below are close enough to overlap. In a ring structure, delocalized electrons are indicated by drawing a circle rather than single and double bonds. Once again, the octet rule must be observed: One of the most common examples of this feature is observed when writing resonance forms for benzene and similar rings.